Background:
There have been significant advancements in the treatment of multiple myeloma (MM) over the last 20 years including an influx of recently approved novel therapies. However, with these advances in treatment, the optimal combination and sequence of agents remain largely unknown especially in relapsed/refractory MM. A major unmet need in MM is patients with high-risk disease. Revised International Staging System is used to identify patients with high-risk MM, however a better definition of high risk includes "functional high risk" (i.e. patients relapsing within 18 months from diagnosis). As it has been previously shown that clinical trial populations are not universally representative of those in the routine practice setting, real-world data (RWD) can provide valuable insight into this rapidly evolving treatment landscape and high-risk population. Here we explore treatment patterns and outcomes of functional high-risk MM, patients who relapse within 18 months of initial diagnosis.
Methods:
This retrospective study utilized the COTA real-world database, a de-identified database of RWD derived from the electronic health records of partnered healthcare providers in the United States. A total of 958 patients were identified as having been diagnosed with active MM between Jan. 1, 2015 and Jan. 1, 2020 and experienced early relapse (defined as relapse within 18 months of initial active MM diagnosis and treatment). Practice setting distribution of this cohort was 84% academic and 16% community. Line of therapy was assigned programmatically utilizing IMWG definitions and guidelines. Patient characteristics and treatment patterns across the first (1L) and second lines (2L) of treatment were assessed using descriptive statistics. Time to next treatment (TTNT) was calculated overall and within treatment subgroups of interest as a surrogate for progression-free survival.
Results:
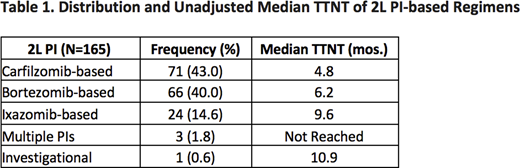
Among this functional high-risk patient population, the mean age was 64 yrs. (SD ±11.3) and the patients were predominantly white (72.6%). The most common cytogenetic abnormalities at diagnosis were del(13) (46.0% positive), 1q (36.4%), and t(11;14) (25.4%). In 1L, the majority of patients received a triplet regimen (75.4%), most commonly proteosome inhibitor (PI) + immunomodulator (IMiD) + steroids (45.0%). A total of 56 patients (5.9%) received stem-cell transplant (SCT) in 1L. Overall, 16.3% of the population did not receive 2L therapy due to death. Among the patients who received 2L therapy after early relapse (N=869), 50.5% received an SCT. TTNT was significantly longer for patients receiving SCT in 2L as compared to those who did not (34.8 vs. 5.8 months, respectively). Among patients who did not receive SCT (N=430), triplet therapy was most common (61.2%) with PI + IMiD + steroids representing the largest regimen group (30.2%). Table 1 shows the distribution of PI drugs within the 2L PI + IMID + steroids group and their associated median TTNT. No significant differences were observed when comparing median TTNT of 2L PI + IMID + steroids to daratumumab-based regimens (5.3 vs. 5.6 months, respectively).
Conclusions:
In our real-world population, median TTNT for functional high-risk patients was 17.8 months. 50.5% of these functional high-risk patients received SCT in 2L with the most common induction regimen containing cyclophosphamide + etoposide + dexamethasone (CED). For patients not receiving SCT in 2L, the most common regimen type included PI + IMID + Steroids. In comparing antibody-based therapy vs. PI + IMID + dexamethasone-based therapy, daratumumab-based combinations showed no significant difference in unadjusted analysis. Our study highlights some very important observations in functional high-risk patients. First, if patients are SCT eligible and it is not performed as part of first line treatment, SCT still provides the best outcomes in regard to TTNT in the second line setting. For patients not receiving SCT, our RWD demonstrates that 30.2% receive PI + IMID + dexamethasone as 2L treatment with only 13% receiving carfilzomib-based combination. Our study highlights the poor outcome of functional high-risk patients and provides insight into treatment patterns in 2L therapy. Further research is needed to explore patient and disease characteristics of functional high-risk patients and study novel treatment strategies like CAR T cell therapy or T-cell engagers in these patient population.
Belli:COTA, Inc.: Current Employment, Current equity holder in private company. Hansen:COTA, Inc.: Current Employment, Current equity holder in private company. Kansagra:Alnylam Pharmaceuticals, Bristol Myers Squibb /Celgene, GlaxoSmithKline, Janssen, Pharmacyclics, Takeda Pharmaceuticals, Pfizer, Karyopharm Therpeutics: Other: Advisory Board. Dilwali:COTA, Inc.: Current Employment, Current equity holder in private company. Wang:COTA, Inc.: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.